Presoak kind of sounds like something extra that only the most dedicated car washer might do. In reality, it’s a crucial part of any car wash process. Regardless of it being self-serve, in-bay automatic, or tunnel, presoaks set the tone for the rest of the wash and must be applied correctly if you hope to achieve a completely professional wash.
Learning about pH balance is critical when dealing with car wash chemicals, and it’s incredibly important with presoaks. Car wash presoak chemicals are typically broken down into low pH and high pH options. Acid and base chemicals target different types of soils on vehicles. One type might be more important depending on your area and customers, but the most effective car washes use a 2-step process with a low pH and a high pH to thoroughly clean a car. Learn about the most effective way to use both types below.
When Should You Use a High pH Presoak?
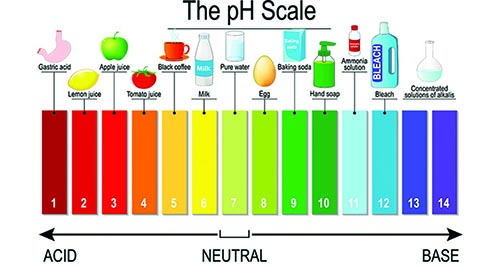
Running from about 8 to 14 on the pH scale, high pH presoaks contain alkaline builders and surfactants that remove organic soils and neutralize acids that may be on a car’s exterior. Bleach, ammonia, sodium hypochlorite, and sodium hydroxide are some examples of chemicals you might see in a high pH presoak.
Examples of Organic Materials Removed by Alkaline
- Road film
- Heavy oils
- Grease
- Fats
- Bugs
- Proteins
- Heavy soil buildup
- Bird droppings
When Should You Use a Low pH Presoak?
Ranging from 1 to 6 on the pH scale, low pH presoaks are acidic formulas that target inorganic materials with high mineral compositions. Oxalic acid, citric acid, and hydrofluoric acid are some examples of chemicals that you might find in a low pH presoak.
Examples of Inorganic Materials Removed by Low pH
- Heavy oxides
- Salts
- Metal particles
- Heavy clay
- Granular materials
- Rust